
Executive summary
The European Union (EU) is advancing the creation of data spaces to foster innovation, enhance data sharing and ensure data sovereignty. Two key legislative pillars in this effort are the European Health Data Space (EHDS) Regulation and the Open Data Directive (ODD).
The EHDS aims to increase the availability, reusability and standardisation of health data systems across the EU, particularly by promoting the interoperability and the security of electronic health records systems. The EHDS complements the ODD, which focuses on making government-held data publicly available for reuse. The combination of data being made available both under the EHDS and the ODD is expected to benefit the various stakeholder groups by providing new opportunities for innovation and data reuse. This report explores the synergies and complementarities between these two EU legislative instruments. It investigates how combining protected health data under the EHDS with open data under the ODD can create new opportunities for various stakeholder groups, including citizens, healthcare professionals, researchers and policymakers.
Through a combination of desk research and expert interviews, the report identifies concrete use cases, benefits and challenges associated with leveraging open and protected data in the context of the EHDS. The findings aim to support the implementation of the EHDS and provide tangible recommendations for enhancing the role of open data in future data spaces.
Patients
- Benefits for patients include the possibility of data-driven choices for healthcare providers that offer lower waiting times and facilities that provide higher quality of care.
- Challenges include the lack of skills, tools and knowledge to process, analyse and derive insights from this data in practice.
Healthcare providers
- Benefits for healthcare providers include additional access to data relating to their field of practice, potentially leading to better diagnosis and treatment.
- Challenges include the potential additional workload in an environment that is characterised by time constraints, and the lack of skills, tools and knowledge available to healthcare providers to analyse data and extract insights themselves. However, this challenge can be mitigated by providing applications that use EHDS-related data and open data and that are integrated into systems used by healthcare practitioners.
Policymakers
- Benefits include increased data-driven policy development and consequentially better health-related (strategic) policy.
- Challenges relate to the lack of skills and the lack of tools available to process, analyse and derive insights from this data in practice.
Reusers (researchers, innovators)
- Benefits for reusers are numerous, such as access to additional data. Moreover, reusers benefit from quicker access to data, which enables faster analysis, decision-making and innovation. Their expertise in data management allows them to make effective use of this access.
- Challenges for reusers include a reluctance to share data, despite the EHDS’s emphasis on data altruism. This may limit the extent to which data becomes available for reuse.
It can be seen that the synergy between the data under the EHDS regulation and the data under the ODD can significantly enhance data-driven innovation in healthcare. This particularly benefits reusers such as researchers, as they are the ones who directly engage with the data. While other stakeholders benefit more indirectly, through the digital tools and services built on top of this data, researchers are positioned to immediately leverage the data for analysis, development and innovation. The provided policy recommendations focus on ensuring that outcomes generated by researchers, such as insights, tools or innovations, are made accessible and usable for other stakeholders, including healthcare providers, policymakers and developers. Additionally, our recommendations call for promoting data literacy and analytical skills among patients and healthcare providers, who primarily use health data for care, to encourage the reuse of EHDS-related data and open data.
To ensure that open data remains visible and effectively integrated within emerging data sharing frameworks such as the EHDS, data.europa.eu should showcase use cases that demonstrate how open data complements protected data, such as combining mobility and health data for public health planning, and provide guidance to stakeholders on best practices for linking and reusing open data within these new infrastructures. It is also recommended to re-evaluate the synergies between the EHDS and the ODD in a few years, once the use of the EHDS infrastructure and the availability of data under its mandate have become widespread in practice.
1. Introduction
The European health data space (EHDS) ([1]) and the Open Data Directive (ODD) (Directive (EU) 2019/1024) ([2]) are both key components of the European Union’s (EU) broader strategy for data ([3]), yet these legislative instruments differ in scope and the types of data they cover. In this report, we aim to explore these differences and complementarities by describing concrete use cases that illustrate how data made available under these two frameworks can be applied in practice.
The EHDS is specifically focused on health data ([4]). Its three main objectives are to (1) increase the availability of healthcare data in an international setting for care purposes (primary use); (2) increase the reusability of healthcare data for research, policy and innovation purposes (secondary use); and (3) increase (technical) standardisation of European electronic health record (EHR) systems to increase cross-border interoperability ([5]). In contrast, the ODD targets a broader range of public sector data, including a list of high-value datasets ([6]), with the aim of making this information openly accessible for reuse by any stakeholder ([7]).
To aid understanding, it is important to first clarify the types of data and access models involved.
- Open data refers to non-personal, government-held data that is made freely available for reuse. This is the focus of the ODD.
- Restricted data includes personal or sensitive data that requires safeguards for privacy and security. This is the domain of the EHDS, which governs access to health-related data such as medical records, insurance claims, clinical trials and data from wearables and health apps.
- Personal/private data is a subset of restricted data and includes identifiable health information.
This distinction also shapes their respective access and sharing arrangements. Under the EHDS, access to data – especially for secondary use – is carefully managed through health data access bodies (HDABs), which evaluate requests and ensure compliance with data protection regulations. In contrast, the ODD promotes open access, encouraging public institutions to make data freely available through portals, with minimal restrictions. A key element of the ODD is Commission Implementing Regulation (EU) 2023/138, which defines a list of high-value datasets that public sector bodies are required to publish. These datasets are grouped into six thematic categories: geospatial, earth observation and environment, meteorological, statistics, companies and company ownership and mobility. The regulation mandates that these datasets be made available free of charge, in machine-readable formats and via application programming interfaces, to maximise their reusability and socioeconomic impact. This framework reinforces the EU’s commitment to data openness by ensuring that the most valuable public sector data is accessible under harmonised conditions across Member States ([8]).
Despite these differences, both initiatives share a common ambition: to unlock the value of data for innovation, transparency and improved services. While the EHDS is more targeted toward healthcare-specific advancements, the ODD supports a wider range of economic and societal benefits. Together, they represent complementary approaches to building a more data-driven EU, with potential synergies particularly in areas where health-related public data intersects with broader datasets under the ODD, albeit combinations like these must be handled carefully to protect privacy (as discussed later in the report).
The EHDS and the ODD also both encourage the use and reuse of data. In this regard, the combination of the EHDS and the ODD might have positive implications for several stakeholder groups (specifically patients, healthcare providers, policymakers and reusers). The combination of data made available under these two frameworks offers new ways for innovation and data reuse. In this report, we explore the possible benefits of the combination of the EHDS and the ODD for several stakeholder groups.
This report investigates the following research question: what are the synergies and complementarities between data made available under the EHDS and data made available under the ODD?
The sub-questions relating to this main research question are the following.
- In terms of primary use (use of health data for patient care) and secondary use (reuse of health data for research, policymaking, etc.), what are the benefits of the EHDS for patients and healthcare professionals, and what barriers exist that may hinder the full realisation of these benefits, particularly in light of the existing ODD?
- In terms of secondary use, what are the benefits of the EHDS for reusers and policymakers, and what barriers exist that may hinder the full realisation of these benefits, particularly in light of the existing ODD?
The logic of these sub-questions is visualised below.
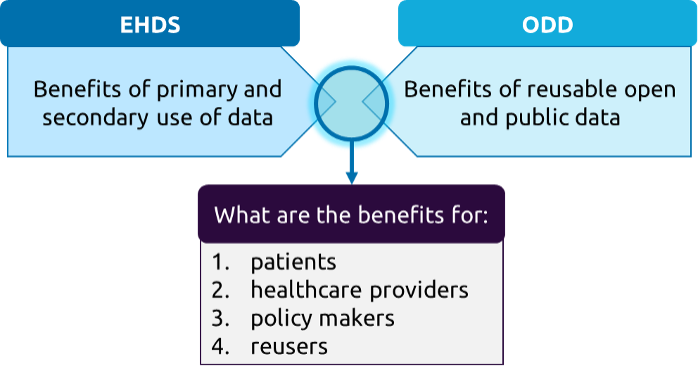
Figure 1: Visual of research scope, author’s creation
The report starts with a concise summary of the policy and user landscape in which the European data strategy, the EHDS and the ODD are introduced. To enrich this landscape, we conducted interviews with experts from various European Member States, representing diverse areas of expertise related to the EHDS and open data. This summary is followed by the research methodology. The report concludes with the findings of the interviews, a set of use cases, recommendations and conclusions. These use cases serve as a central component of the research, offering concrete – though in some cases still conceptual – examples of how data sharing under the EHDS can generate value. They are intended to illustrate the potential benefits of the EHDS and the ODD in practice and to demonstrate how the frameworks can support innovation, improve services and inform future policy development.
2. Policy landscape: how open data interacts with the EHDS
2.1. A European strategy for data
The European strategy for data ([9]), launched by the European Commission in 2020 ([10]), is a cornerstone of the EU’s digital transformation agenda. One of its goals is to create a single market for data, where data can flow freely across sectors and Member States, while ensuring high standards of privacy, security and ethical use. As part of building a single market for data, common European data spaces ([11]) are being created in a number of strategic areas. These data spaces are supported by a suite of key legislative instruments that complement domain-specific efforts like the EHDS.
- The Data Governance Act (Regulation (EU) 2022/868 ([12])) establishes frameworks for data sharing, including data altruism and data intermediation services, and sets up the European Data Innovation Board.
- The Data Act (Regulation (EU) 2023/2854 ([13])) clarifies rights and obligations around access to and use of data, especially data generated by connected devices, ensuring fairness across the data economy.
These data spaces are a means to bring together relevant data infrastructures and governance frameworks to facilitate data pooling and data sharing.
2.2. The European health data space
In March 2025, the European Health Data Space Regulation ([14]) (Regulation (EU) 2025/327) was officially published in the Official Journal of the European Union ([15]). The EHDS aims to enhance citizens’ access to and control over their health data (primary use) at both the national and the EU levels. The regulation seeks to establish a legal framework with trusted governance mechanisms and to ensure a secure environment for data processing. Additional goals include supporting the free movement of citizens by ensuring their health data accompanies them and fostering a unified market for digital health services and products. By involving citizens in their healthcare, the regulation aims to improve the quality and continuity of care throughout the EU. Furthermore, standardising rules and obligations for the interoperability and security of electronic health record systems is expected to reduce costs associated with the flow of health data across the EU.
Moreover, the EHDS aims to facilitate the reuse of health data (secondary use) for research, innovation, regulatory and public policy purposes across the EU. The secondary use of health data is expected to provide efficiency gains for data users in the health sector. Creating a single market for data will allow it to flow freely within the EU and across sectors for the benefit of the public, businesses, researchers and public administrations for purposes such as policymaking, research and innovation. Considering that a substantial amount of electronic data to be accessed in the EHDS is personal health data relating to natural persons in the EU, the EHDS was designed in full compliance with the General Data Protection Regulation (Regulation (EU) 2016/679) ([16]).
The EHDS will be rolled out through a combination of technical and organisational measures. The data space will build on existing infrastructure like MyHealth@EU ([17]) and promotes the development of interoperable national platforms. The EHDS aims to overcome the fragmentation of current health data sharing practices across the EU. By building on existing infrastructure like MyHealth@EU and promoting interoperable national platforms, the EHDS aims to create a unified framework that enables secure, cross-border access and reuse of health data. It empowers patients, supports innovation and research, ensures compliance with data protection through HDABs and enhances the efficiency and resilience of healthcare systems, transforming isolated data silos into a cohesive, patient-centric and innovation-driven ecosystem.
The EHDS will not be an entirely open-access platform. Instead, it will operate under a tiered access model.
- Patients will have faster, free and secure access to their own electronic health data.
- Healthcare professionals will access patient data for treatment purposes only, with patient consent and under strict data protection rules.
- Reusers (e.g. researchers, public authorities, innovators) will be able to request access to anonymised or pseudonymised data for secondary use through HDABs. These bodies will evaluate requests and ensure compliance with ethical, legal and technical safeguards.
The EHDS is not a single database but a federated system of connected platforms, governed by harmonised rules and supported by robust technical standards to ensure trustworthy, secure and equitable access to health data across the EU. The rollout is supported by EU-funded projects such as EU4Health ([18]), Horizon Europe ([19]) and TEHDAS2 ([20]), which help define technical standards and pilot real-world applications. Each Member State will also establish a HDAB ([21]) to manage and authorise secondary data use, ensuring compliance and secure sharing15.
2.3. The Open Data Directive
The directive on open data and the reuse of public sector information (Directive (EU) 2019/1024) ([22]) provides common rules for a European market for government-held data. One of the main aims of the open data directive is to make public sector data (e.g. from public sector bodies in Member States, at the national, regional and local levels) and publicly funded data (e.g. from meteorological institutes) available for reuse. The directive also required the adoption of an implementing regulation regarding high-value datasets (HVDs) (Commission Implementing Regulation (EU) 2023/138) ([23]). HVDs are certain public sector datasets that have been identified as having important benefits for society, the environment and the economy across six categories: geospatial, earth observation and environment, meteorological, statistics, companies and mobility. Notably, ‘health’ is not one of the HVD categories, reflecting the sensitive nature of personal health data and the decision to handle it via specific frameworks like the EHDS. The HVD regulation strengthens the EU’s open data ecosystem by applying the FAIR principles (findable, accessible, interoperable, reusable) and harmonising reuse conditions across Member States, thereby reducing legal and technical barriers to data-driven innovation.
On data.europa.eu, the official portal for European data, there are 26 896 datasets categorised as ‘health data’ (as at 3 January 2025) ([24]). Open data related to healthcare could include for example data on spending, aggregated data on vaccination coverage, hospital admissions and discharge rates, hospital performance indicators (wait times, readmission rates) and so on ([25]). Open data in healthcare can have a significant impact:
- it can contribute to enhanced public health outcomes by enabling early detection of disease outbreaks and supporting evidence-based policymaking;
- it promotes transparency and accountability through access to spending and performance data;
- it drives innovation and research by fuelling data-driven discoveries and AI development;
- it empowers citizens to make informed healthcare decisions; and
- it improves operational efficiency by helping providers benchmark performance and optimise resources ([26]).
2.4. Relationship between the Open Data Directive and the European health data space
Since both the ODD and the EHDS are part of the European data strategy, a complementary relationship is expected. For example, both the ODD and the EHDS emphasise the benefits of making data available for reuse. Their complementarity lies in the conditions under which data can be shared. Open data covered by the ODD concerns aggregated health statistics that are non-sensitive and broadly reusable, while the EHDS governs access to individual-level health data under strict conditions for authorised secondary uses such as research. The ODD facilitates broad public access and innovation through open licensing, whereas the EHDS introduces a controlled environment with governance, consent and security mechanisms tailored to sensitive health data ([27]). Both initiatives aim to foster a culture of data sharing across the EU. The ODD does this by promoting open data practices among public institutions, while the EHDS relies on data altruism; individuals voluntarily making their health data available. This shared cultural ambition – to normalise and encourage responsible data sharing – highlights a key area of convergence between the two frameworks and lays the groundwork for addressing the cultural and institutional barriers that may hinder their implementation.
Together, these regimes form a layered approach to data sharing. For example, aggregated health statistics published under the ODD can be combined with granular, individual level data accessed through the EHDS to support more robust and innovative analyses. This creates a synergy between restricted data under the EHDS and open data. Specifically, open data made available by public institutions can play a foundational role in powering a wide range of data-driven applications, including those that will draw on additional data shared through data spaces.
Each framework offers distinct benefits and opportunities for different target audiences.
- Patients benefit from greater transparency and empowerment. The ODD ensures public access to health-related statistics and environmental data, while the EHDS gives individuals control over their personal health data and the ability to contribute to research.
- Healthcare professionals gain access to cross-border patient data, improving continuity of care. This also helps continuity of care within Member States, since not every Member State currently has good access to patient data.
- Researchers can combine open datasets with controlled access to health data through the EHDS to develop more precise models, tools and interventions. This supports data-driven innovation.
- Policymakers can use open data for transparency and accountability, while leveraging EHDS data for evidence-based policymaking, public health planning and crisis response.
The interaction between the ODD and EHDS raises some risks that require mitigation. Combining open data with data accessed through the EHDS could potentially increase the chance of identifying individuals. Open data is typically anonymised and aggregated, but it may still contain quasi-identifiers (e.g. age range, region, diagnosis codes) that, when combined with more granular data from the EHDS, could narrow down the identity of individuals. Data shared under the EHDS, while accessed under strict governance and for authorised secondary use, may include pseudonymised individual-level health data. If a reuser has access to both datasets, they might be able to cross-reference them to infer identities – especially in small populations or rare disease cases. However, this is provided for in the legislation and is strictly forbidden under the EHDS (Article 61).
3. Key stakeholders in data sharing under the European health data space and the Open Data Directive: roles, interests and impact
In the context of the EHDS and the ODD, several user groups play an important role in data sharing practices. To ensure a focused and meaningful analysis, this section concentrates on the key user groups that are most directly involved in or affected by these legislative frameworks. A fundamental distinction throughout this section is made between primary use – the use of data for the original purpose for which it was collected, such as direct patient care – and secondary use, which refers to the reuse of data for other purposes such as research, policymaking, innovation and public health planning. This distinction is essential for understanding the different ways in which various user groups engage with and derive value from data sharing under the EHDS and ODD, and it frames the analysis that follows.
The selection of user groups in this report – patients, healthcare providers, policymakers and reusers –reflects a deliberate effort to combine the core target audiences of both legal frameworks. These groups represent the intersection of those most impacted by the EHDS’s governance of sensitive health data and those who stand to benefit from the ODD’s open data ecosystem. This combined perspective allows for a more comprehensive understanding of how both frameworks contribute to a shared goal: fostering a culture of responsible and impactful data sharing across the EU.
3.1. Patients
Patients (citizens in their role as recipients of care) are the primary end users, benefiting from both primary and secondary uses of health data. Through the EHDS, they gain direct advantages such as improved access to their health records and better continuity of care, especially across borders via systems like MyHealth@EU. Indirectly, they benefit from secondary uses of anonymised data, which support research and innovation, leading to new treatments, diagnostic tools and personalised care. The EHDS ensures this is done securely, with strong governance and consent mechanisms. Meanwhile, the ODD complements this by making aggregated health statistics openly available, enhancing transparency and public awareness. Together, these frameworks empower patients and place them at the heart of a responsible, data-driven healthcare ecosystem.
3.2. Healthcare providers
Healthcare providers play a central role in the exchange and use of health data. They directly interact with patients and utilise health data for primary purposes such as diagnosis, treatment and the optimisation of care. Additionally, they benefit indirectly from secondary use of data: if research leads to new medical technologies, improved treatment protocols or healthcare innovations, providers can enhance their services through more efficient workflows, reduced operational costs and access to improved tools for diagnosis and treatment. For example, AI-powered clinical decision support systems trained on anonymised datasets accessed through the EHDS could help physicians identify rare diseases more quickly or recommend personalised treatment plans based on real-world evidence. This not only improves patient outcomes but also supports healthcare professionals in making more informed, data-driven decisions.
3.3. Policymakers
Policymakers shape the regulatory frameworks and guidelines governing the use and exchange of health data. Their decisions impact primary use by ensuring that the ways data are handled align with ethical and legal standards. Policymakers also influence secondary use by promoting responsible data reuse for research and innovation. If data-driven policies lead to improved healthcare systems, accessibility or patient rights, the broader population, including patients, benefits from these advancements.
3.4. Reusers
Reusers encompass researchers, data analysts and organisations that leverage health data for secondary purposes, such as scientific studies, technological development and improvements to public health. Their work may not involve direct interactions with patients, but it contributes to innovations that enhance healthcare delivery. Reusers benefit from access to data because they can create products and services that would otherwise not be possible, e.g. through new business models.
4. Results: advantages and challenges related to the interaction of EHDS data with open data
Through both literature research and analysing the interview data (see Annex A for the full methodology), we have extracted several key conclusions per end user group. We have described the advantages and challenges that are experienced by patients, healthcare providers, researchers and policymakers. Each section starts with a description of the benefits of combining data shared under the ODD and EHDS and concludes with the challenges that may arise. Firstly, for clarity, we have included a table that summarises the different benefits and challenges for each user group.
| Patients | ||
| This user group is comprised of the general group of patients who receive a form of healthcare in a medical facility. | ||
| Nature of the benefit or challenge | Summary description | |
| Benefits | Patients can make better informed outcomes. | Increased ownership of healthcare data from the EHDS and access to open data can enable patients to make better informed outcomes. This leads to choices for facilities where they perceive additional benefits, such as higher quality of care or lower waiting times. |
| Challenges | Patients currently lack the skills, tools and knowledge to combine their data. | Patients currently, in general, do not have access to or are not able to find and use the right tools, skills and knowledge to effectively combine EHDS data and open data. |
| Patients lack incentives to combine EHDS data and open data. | In general, the broad user group of patients is not incentivised to combine different sources of data. An exception is the subgroup of patients who are motivated to and experienced in managing their own health (e.g. those with rare diseases). | |
| Healthcare providers | ||
| This user group is comprised of professional healthcare providers such as doctors in a medical facility. | ||
| Nature of the benefit or challenge | Summary description | |
| Benefits | Healthcare providers might be able to increase the quality of the care they provide. | When healthcare providers gain access to tools that combine data from the EHDS with other sources of open data, they can benefit from these insights to improve their quality of care. |
| Challenges | A significant (time) investment is seen as necessary. | To make effective use of EHDS data and open data, investment of time and resources is required, which is not always possible for this end user group. |
| Skills to combine data and extract insights are currently lacking. | Healthcare providers, in general, do not always possess the right skills and knowledge to effectively use and directly benefit from the combination of data from the EHDS and open data. | |
| The right tools are missing. | There are currently no, or limited, tools available that provide a user-friendly way (such as integration into an EHR system) to let healthcare providers benefit from the combination of open data and data from the EHDS. | |
| Policymakers | ||
| This user group refers to policymakers in a general sense, including local, regional, national and international policymakers. | ||
| Nature of the benefit or challenge | Summary description | |
| Benefits | Additional options for data-driven policymaking become available. | Policymakers can combine data under the EHDS and other open data to generate insights valuable for policymaking. |
| Challenges | Skills to combine data and extract insights may be lacking. | In general, policymakers may not have access to or are not sufficiently equipped to effectively benefit from using data from the EHDS in combination with other sources of open data. |
| Researchers/reusers | ||
| This user group refers to researchers in a broad sense, be it from an academic perspective or other research institutions. | ||
| Nature of the benefit or challenge | Summary description | |
| Benefits | Greater availability and accessibility of data can lead to better research outcomes. | Researchers can greatly benefit from the combination of data from the EHDS and other open data, as they are, in general, equipped with the right tools and skills to make use of this data. |
| Challenges | Although the EHDS promotes data altruism, the culture around data sharing is not yet fully developed. | Although the EHDS contributes to additional availability of data, there are still concerns regarding the operationalisation of ‘data altruism’. The concern centres around the lack of willingness to share data more broadly. |
| The scope of data availability and discoverability is good, but not yet complete. | The EHDS will improve data availability and discoverability at the EU level, but lacks global coverage, which inherently might lead to incomplete data availability. | |
Below we further elaborate the benefits and challenges for each user group.
4.1. Patients
4.1.1. Benefits
A. Patients can make better-informed decisions on healthcare providers
A recurring benefit identified through the interviews is that patients can make better-informed outcomes in terms of selecting healthcare institutions where they receive healthcare. For example, patients may research facilities that promise lower waiting times for planned medical care or greater quality of care (that is, use public/open data sources on healthcare facilities or websites from healthcare institutions to make informed decisions about the healthcare provider). Under the EHDS, the patient’s health data is easily shared between providers to deliver healthcare (primary use).
4.1.2. Challenges
A. In general, patients currently lack the skills, tools and knowledge to combine their data
Patients who require care often expect to receive it from their current healthcare provider. Through both desk research and interviews it was found that patients, in general, may lack the right skills, tools and knowledge to effectively combine their own medical data with other open data. Even when patients have sufficient digital literacy to access data, analysing the data to retrieve valuable and impactful insights is an additional challenge that requires even more advanced skills, tools and/or training. Open data, especially in the form of open datasets, can be too technical for many patients to effectively work with ([28]). Indirectly, this challenge could be mitigated by (private sector) organisations who do have the skills and tools to combine data from the EHDS and other open data, who can share their insights with patients.
B. Patients are lacking an incentive to combine EHDS data and open data
Although in theory patients can combine their online accessible EHDS data and open data to gain insights into better healthcare facilities and shorter waiting lists, for example, it has been noted that a clear incentive for patients to combine data is still lacking. As patients are, in general, at the receiving end of care, it is unlikely that patients will actively research their own EHDS data and combine this with other open data.
It is worth stressing that this observation does not represent the whole patient end user group. There might be patients who are in practice willing and able to combine their own healthcare data with other sources to research possibilities for better personal health outcomes, especially chronically ill patients or patients with rare diseases. In these cases, patients could make use of digital platforms such as the PatientLikeMe concept ([29]) to share their data to gather more insights about a specific disease or disease trajectories.
4.2. Healthcare providers
4.2.1. Benefits
A. Healthcare providers might be able to increase the quality of care they provide
Primarily, interviewees acknowledge that the EHDS can and will have strong benefits in their daily practice. It allows for easier and faster access to patient-related data that can increase the quality of healthcare. This strongly relates to the primary use side of the EHDS, which will be facilitated through EU-wide digital infrastructure.
When researching the benefits of combining data from the EHDS with other open data, it was noted that when healthcare providers receive the right tools, they can benefit from this combination of data. These tools can help healthcare providers increase their quality of care if these tools have a high degree of usability and are preferably integrated into EHR systems. This would allow healthcare providers to benefit from insights from this combined data.
4.2.2. Challenges
A. A significant (time) investment is seen as necessary
This report finds that a challenge for this end user group is the time investment needed for them to combine healthcare data under the EHDS with other open data. Specific examples include experts who mention that doctors in general have approximately 10 minutes per patient to provide actual care, which leaves no time for performing analyses that require combining data that directly leads to better outcomes in terms of healthcare. One interviewee stated that it is not reasonable to expect healthcare professionals to combine data from the EHDS and open data in practice, as they might be not able to do so in a time efficient way.
Furthermore, generating usable and reliable health data requires significant investments. This includes investments in technology, infrastructure, staff training and ensuring the security and privacy of the data ([30]).
B. Skills to combine data and extract insights are currently lacking
Another barrier that has emerged from the research is that the skills that are required to combine data from the EHDS and other open data are lacking. Although availability may not be an issue since open data is readily available, the specific tools required to combine data and gather relevant insights is a barrier. This report finds that while combining data requires specific skills, it is also difficult to ensure high quality outcomes. Currently, it is unclear for this end user group how to assess the outcomes of combined data.
A specific example relates to the complexity surrounding healthcare data standardisation. Although there are common standards, such as the health DCAT-AP (data catalogue vocabulary application profile for data portals in the EU) standard for describing the metadata of datasets ([31]), a lack of skill and knowledge of this standardisation limits its effective usage.
C. The right tools are missing
One interviewee mentioned that there is currently a lack of understandable tools available to healthcare providers for combining data. Even if healthcare professionals wish to combine data and have the required time available, they still lack the specific tools required to do so. The EHR system has been identified as a tool that is potentially helpful. This is already an important source of patient data. Additional, specific tools could also be made available within these systems to allow healthcare providers to experience clear benefits.
4.3. Policymakers
4.3.1. Benefits
A. Additional options for data driven policymaking become available
Policymakers are frequently mentioned as important beneficiaries of the EHDS ([32]) and of the combination of EHDS data and other open data. By combining multiple sources of data, insights might be generated that lead to better policy development and policy decisions. Yet, in both desk research and interviews, it was mentioned that this is currently still a theoretical scenario, since it is not expected that policymakers will be directly able to process and analyse this combination of data.
In practice, for effective secondary use of data, policymakers will have to send in a data request or request a permit for data access as required under the EHDS ([33]). This requires that policymakers have a thorough understanding of the requirements needed to gain access to the data and that they have the skills to further combine this data and extract insights. A practical example of combining protected health data with open data given by interviewees is the combination of health data and environmental data to assess health outcomes. These kinds of combinations can be valuable for policymakers but require skill to execute in practice.
4.3.2. Challenges
A. In general, policymakers currently do not have the right skillset
Our findings indicate that there is a specific data-related skillset required to combine data and extract insights. The simple act of effectively combining data is already complex, let alone extracting insights from this combined data. The interviews indicate that it may be necessary for policymakers to gain these new skills.
4.4. User group: reusers
4.4.1. Benefits
A. Higher availability and accessibility of data can lead to better research outcomes
Our results indicate that out of all the end user groups, reusers and especially researchers will benefit the most from access to data under the EHDS combined with other open data. In both desk research and the interviews, it was indicated that researchers tend to have the right skillset and tools available to combine this data and extract insights. Also, in terms of time, researchers and other data users have the greatest possibilities to make optimal use of combined data since their focus is already on this topic. Currently, many researchers already combine multiple datasets for research purposes. The EHDS will enable the upscaling of this process by offering faster and easier access to more data sources.
Examples mentioned by interviewees include research on the combination of environmental data and healthcare data. For example, a project called Green Data for Health ([34]) in France uses open data to research the impact of environmental noise on sleep, in combination with prescription drugs, for over 10 million inhabitants. Besides this example, the Green Data for Health project has several studies in which they combine environmental data with health outcomes ([35]).
4.4.2. Challenges
A. Although the EHDS promotes data altruism, the culture around data sharing is not yet fully developed
A challenge that is mentioned is the current culture around data. Interviewees expressed the sentiment that in the general community of data holders and researchers, there is still a strong emphasis placed on data protection, leading to reluctance to share data freely. This reluctance could lead to lower eventual availability of healthcare data. On the other hand, the EHDS mandates that data holders make their data available for reuse purposes. Over time, as the legal requirements become practice and successful examples of data use accumulate, the culture might change and the reluctance around data sharing may diminish.
B. The scope of data availability and discoverability is good, but not yet complete
Interviewees mentioned that the increasingly standardised digital infrastructure that is to be provided under the EHDS will further improve data availability and data discoverability. Yet, it was also mentioned that although this will ultimately lead to better data availability on a EU scale, researchers may also frequently carry out research that is global in scope. Some interviewees identified this as a challenge in terms of using data from the EHDS.
C. Differentation in the maturity of how EHDS processes are adopted
As the EHDS has entered into force, Member States are starting to initiate different processes to fully apply the EHDS. This requires the development and implementation of digital capabilities, such as the HDABs for secondary use of data. An issue identied in the interviews is the possible disparities in terms of the maturity of EHDS processes in Member States. Several Member States that participated in the interviews, such as Portugal and France, have already made significant efforts to technically develop and implement the capabilities required for primary and secondary use. It is expected that other Member States may be behind in terms of maturity, which may now and in the (near) future limit the usability of EHDS-related data and other open data for reuse.
5. Use cases, recommendations and conclusions
Throughout the interviews and the desk research, several use cases were discovered and discussed. We have summarised several of the use cases below. After that we have provided policy recommendations, before ending with our conclusions.
5.1. Use cases
The use cases mentioned below are formulated to illustrate clear examples of the benefits that can arise from combining both EHDS-related data and other sources of open data. We separate use cases related to the primary use of data, which mostly benefit patients and healthcare providers, and use cases related to the secondary use of data, which mostly benefit researchers and other reusers.
Use cases related to the primary use of data
- Use case 1 – citizens
An Italian citizen is on the waiting list to undergo a surgical procedure. The waiting list is over six months. Determined to find a solution, he began researching hospitals across Europe with shorter waiting times by accessing open data sources ([36]). His search led him to a renowned hospital in Austria, known for its excellent healthcare services and shorter waiting periods. The hospital in Austria could access his health data to perform the surgery, as this is facilitated through the EHDS. All the information on the procedure is available in Italy through the same EHDS mechanism, in the electronic healthcare record system. Combining his own personal data on the specific medical condition with open data on healthcare quality and having international access to data is a relevant use case and improves the experience for the patient and the quality of healthcare.
- Use case 2 – healthcare providers
Healthcare providers who deliver care in clinical practice benefit from the ability to access healthcare data faster and easier under the EHDS. Also, in general, practitioners face time constraints when it comes to activities that do not relate to the primary functions of care delivery. By providing practitioners with the right digital tools and applications that can effectively combine EHDS-related data from primary use with other sources of open data, they can benefit from the complementarities between EHDS-related data and open data. A requirement of such tools and applications is that they are integrated into the electronic healthcare record system to allow for quick access and user-friendly usage of these tools in the daily activities of healthcare practitioners.
Use cases related to the secondary use of data
- Use case 3 – researchers
In France, a project called Green Data for Health ([37]) is utilising open-source environmental data to assess healthcare effects. The goal of the project is to investigate the impact of environmental noise on the sleep of approximately 10 million French residents. The project exemplifies a powerful synergy between two distinct data sources:
- open environmental data– publicly available noise statistics which are used to map and quantify environmental noise exposure across regions, and
- sensitive health data – prescription data for sleep medications, securely accessed via the Health Data Hub, which provides insights into how individuals manage sleep disturbances potentially linked to noise.
This research combines these datasets and aims to uncover correlations between environmental noise statistics and sleep-related outcomes. The project is a scientific research initiative, jointly managed by the French Ministry of Healthcare and the French Health Data Hub, which is the HDAB in France.
- Use case 4 – policymakers
Mr Dorent, a policymaker in Germany, is committed to improving healthcare outcomes for diabetes patients, whose conditions vary widely due to genetic, lifestyle and environmental factors. To formulate effective policies, he requires comprehensive health data from diverse populations across Europe. However, collecting this data is often complicated by national regulations, fragmented data sources and interoperability challenges. The EHDS mitigates these issues by providing Mr Dorent with access to anonymised health data from patients across multiple Member States. This data, combined with open data from Eurostat, enables him to gather the necessary information to develop informed policies that support personalised treatment plans and enhance healthcare delivery.
These use cases, though simplified, illustrate tangible ways in which the EHDS (with the help of open data) could benefit different end users, from individual patients to researchers and policymakers, once the frameworks are operational.
5.2. Recommendations
- Stimulate research on the intersection of EHDS and open data through funding
As our interviews and desk research indicate that there are several end user groups who can in general benefit significantly from EHDS-related data and other open data, it is recommended that these groups of end users are incentivised to share their results with other user groups. For example, researchers who combine EHDS-related data and other open data should be encouraged and funded to translate their findings into better quality standards for treatment or into innovative treatment protocols. This way, the end users who are in general not able to combine EHDS-related data and other open data (e.g. patients) can benefit from the skills of other end users.
In addition, through this process, the EU can encourage further use of open data and EHDS-related data by sharing best practices across Member States and/or could appoint open data ambassadors who upskill, educate and facilitate interested end users in combining open data with EHDS-related data. Open data ambassadors could be persons or legal entities who have demonstrated their capability to combine EHDS-related data with other open data for better healthcare related outcomes. In addition, open data best practices for data.europa.eu can also be shared more widely. These best practices can inspire and guide users into exploring new combinations of EHDS data and other sources of open data.
- Assess if and how the availability of EHDS-related data in combination with open data can be of direct use for healthcare providers and patients and provide tools to facilitate this
Although the experts interviewed indicated that it is unlikely that healthcare providers and patients will directly combine and analyse EHDS-related data and open data themselves, it may be the case that healthcare providers and patients currently lack the tools and skills to combine this data and therefore have not expressed a strong interest in doing so or have not been able to combine and use data. Therefore, one recommendation is to promote data literacy among these user groups to promote the use of data. If these end user groups do in fact show interest in making direct use of EHDS-related data and open data, tools can be provided that guide and support healthcare providers and patients in combining data and getting valuable insights.
- Provide support and facilitate good governance through education and regulatory bodies
Governmental supervisory and educational bodies can play an essential role in strengthening the knowledge base and skill set of several end user groups. As in the coming years several technical entities and capabilities under the EHDS become operational (for example the HDABs and the health data authorities of Member States), it is essential that end user groups are educated on how these entities will facilitate them in having better and easier access to health data. In addition, it is valuable to continuously educate users on the availability of open data and how this can be combined with EHDS-related data. This should be done under clear governance frameworks that provide guidelines for data protection and security, ensuring end users that their health data is protected.
- Follow developments and reassess later
In the period of 2025–2031 additional requirements of the EHDS will enter into force. Alongside this, the digital capabilities under the EHDS will become operational and will allow for real user interaction. As end users start using these capabilities and start accessing EHDS-related data, it is recommended that periodic evaluation take place on how this data is used and how users combine this EHDS-related data with other open data. t is advisable to monitor the realisation of the technical capabilities across Member States in the coming years and re-evaluate some of the research questions addressed in this report once the maturity of these areas has advanced. It is advisable to do this for example at the end of 2026 or at the beginning of 2027 as EHDS obligations enter into force ([38]).
5.3. Conclusions
The combination of data that will become available under the EHDS with other open data holds substantial potential for advancing data-driven innovation and healthcare in the EU. Under the EHDS, primary use and secondary use of data will increase data access and reusability for several end user groups. In addition to this, these user groups have access to other sources of open data which allow for even greater insights. Below, we summarise how each end user group can benefit from the combination of EHDS-related data and other open data and what possible challenges might arise.
In terms of primary use, patients and healthcare providers can benefit from EHDS-related data in combination with open data. Patients who are willing and able to combine their own healthcare data with other open data sources may be able to make more informed decisions in terms of better healthcare outcomes by, for example, researching options for lower waiting times or facilities that are rated higher in terms of quality of care. A challenge for this end user group is the access to the right skills, tools and knowledge to do this in practice. On the other hand, healthcare providers can benefit through access to applications that combine EHDS-related data and open data and that can be integrated into healthcare practitioners’ daily work. The challenges for this end user group, in general, are the lack of tools and perceived time constraints to understand and use the tools.
In terms of secondary use, the main beneficiaries of EHDS-related data in combination with open data are researchers who are equipped with the right skills, tools and knowledge. In general, possession of these skills, tools and knowledge allow researchers to find, access and reuse data that is made available to them. For policymakers, these benefits are similar, but our results indicate that there is a stronger need for education, skills training and knowledge improvement for this end user group. Of course, other end user groups can benefit from the innovations of secondary use achieved by researchers, policymakers and other reusers.
6. Acknowledgements
This research report has been made possible through the valuable contributions of many stakeholders. Below is a list of organisations that have contributed and wished to be acknowledged. Besides these public acknowledgements, we would like to express our sincere appreciation to all the other stakeholders that participated in this report:
- Digital Health Unit of the European Commission’s Directorate-General for Health and Food Safety,
- Health Data Hub France,
- Integrating the Healthcare Enterprise,
- MyHealth@EU Ireland,
- Nederlandse Federatie Universitaire Medische Centra (Dutch Federation of University Medical Centres),
- Sciensano Belgium,
- Serviços Partilhados do Ministério da Saúde (Ministry of Health in Portugal),
- Ministerie Volksgezondheid, Welzijn en Sport (Ministry of Health in the Netherlands).
Annex A: Methodology
To explore how the EHDS and the ODD can support data-driven innovation and benefit various stakeholders, we applied two main research methodologies: literature research and structured expert interviews. Literature research was used to gather existing insights and policy context, while expert interviews allowed us to collect in-depth, practice-based perspectives from professionals across the EU with diverse expertise in health data and open data. In addition, use cases developed under the EHDS were investigated through the interviews to ground the discussions in real-world applications and assess their relevance and impact.
A.1. Literature research
The findings are based on a structured desk research methodology designed to identify and analyse use cases that illustrate the value of data sharing within the frameworks of the EU’s data strategy, the EHDS and the ODD. This approach involved targeted exploration of practical implementations and stakeholder experiences. Search terms such as ‘European health data space use cases,’ ‘Open Data Directive implementation’ and ‘EU data strategy applications’ were used across platforms like Google, Google Scholar and institutional websites. The research prioritised authoritative sources, including official European Commission materials, national portals on data exchange, outputs from initiatives like TEHDAS and TEHDAS-2 ([39]) and relevant scientific literature. Use cases were selected based on their relevance to the legislative frameworks, clarity of outcomes and demonstrable value creation, with particular attention to examples involving cross-border collaboration, secondary use of health data and innovative applications of open data. This method ensured that the findings are grounded in real-world practices and reflect a broad spectrum of perspectives across the EU.
A.2. Expert interviews
Expert interviews were conducted for deep insights into the interactions between the EHDS and the ODD, with a particular focus on identifying concrete or conceptual use cases that demonstrate the potential benefits of data sharing. The selection of the experts for the interviews was based on the requirement that they have deep material understanding of both the EHDS and the ODD. This was assessed by researching experiences and (research) contributions on these topics. Also, experts that contributed to the development of the EHDS or the ODD were included.
Experts included in this study are categorised into four categories to ensure diverse viewpoints and diverse expertise. The four categories were designed to represent the spectrum of relevant end user groups:
- EU/national policymakers,
- technology specialists,
- healthcare providers/patient associations,
- public- and private-sector researchers/academics/innovators and reusers.
Interviews lasted about one hour and were transcribed. After the interview, the interviewee had the opportunity to review the notes. The interview guideline is included in Annex B of the report and focused on the impact of combining data made available under the EHDS and ODD for several end user groups. Feedback from the interviews is presented anonymously in this report. To ensure a broad and informed perspective, experts were drawn from a diverse range of organisations involved in digital health and data governance across the EU. These include Integrating the Healthcare Enterprise, Sciensano Belgium, MyHealth@EU Ireland, Health Data Hub France, the Digital Health Unit of the European Commission’s Directorate-General for Health and Food Safety, Serviços Partilhados do Ministério da Saúde (Portugal), the Dutch Ministry of Health, Welfare and Sport and the Dutch Federation of University Medical Centres. In total nine interviews were carried out; a summary of participants and/or participating organisations can be found in the acknowledgement section.
Annex B: Interview topic list
General questions
- What topics concerning the EHDS and ODD are you involved in?
- To start with the end user groups, we identified the next end user groups (citizens, healthcare professionals, private sector organisations, policymakers, and researchers). Are they complete? And with which end user group are you most involved in with your organisation?
- In general, do you see any positive use cases for patients when combining the EHDS and the Open Data Directive?
- If yes, what do these use cases look like?
- Do you foresee benefits on the national level from the EHDS and/or in combination with the ODD?
- Specifically for the end user group the interviewee is interacting with a lot.
- Do you see any barriers that may hinder the full realisation of these benefits – particularly in light of the existing Open Data Directive?
- Do you have any policy recommendations for the European Commission on the intersection of the EHDS and ODD?
- Any specific ethical challenges?
- Would you like to address any other topics?
- Can we mention you or your organisation in the acknowledgements?
Depending on the specific expertise, questions about a specific end user group were asked.
Questions specific to patients
- Will patients be able to combine EHDS data with other data? For example would they be able to compare organisations internationally on waiting times?
- How will EHDS change the way citizens interact with their health data?
- What are concerns for citizens, and how are these addressed?
- One of the goals of the EHDS is to give the patient more control of their patient data: how is that done?
Questions specific to healthcare professionals
- Will healthcare professionals be able to combine data?
- How will the EHDS impact healthcare professionals regarding the primary use of data?
- How will EHDS change the way professionals access and use patient data?
- How useful is it for professionals to access patient data from other EU countries?
- Do you see EHDS as a time-saver or an additional administrative burden?
- Will the way of working change when accessing and supplying data cross-border? (In terms of how to register, systems, etc.)
- What challenges do you expect when interpreting health records from different countries?
- Do you foresee any ethical dilemmas regarding access to patient data?
- What challenges might arise for healthcare professionals in terms of training or adapting to EHDS?
Questions specific to researchers and reusers
- How can, in general, reusers benefit from the combination of the EHDS and ODD?
- Do you think the EHDS alone, or the ODD alone, has already benefitted reusers of data?
- Does it seem realistic that reusers combine the EHDS and the ODD to their full extent for their benefit?
- If yes, how can they do this and what would use cases look like?
- If not, what are challenges for reusers that prevent them from using the full benefits from the EHDS in combination with the ODD?
- Are there any other challenges that might arise in:
- the use of the EHDS and the ODD combined,
- the general use of the EHDS,
- the general use of the ODD?
Questions specific to policymakers
- For what end goals can policymakers and regulators use data?
- How do governments directly benefit from the ODD x EHDS?
- Is it realistic that policymakers will directly be able to combine information from data spaces and the ODD to make their work easier?
Glossary
| Term | Definition |
| Data altruism | A concept introduced in EU data legislation where individuals voluntarily make their data available for the common good, such as for research or for public interest purposes. |
| European health data space (EHDS) | An EU initiative aimed at enabling secure access to and sharing of health data across EU Member States for both primary (care) and secondary (research, policy) uses. |
| Health data access body (HDAB) | National bodies responsible for managing access to health data for secondary use under the EHDS, ensuring compliance with legal and ethical standards. |
| Healthcare providers | Professionals and institutions that use health data for diagnosis, treatment and care coordination. They benefit from improved data flows and cross-border access. |
| High-value datasets (HVDs) | Public sector datasets identified under Commission Implementing Regulation (EU) 2023/138 as having significant value for society and the economy, required to be made available under open data conditions. |
| Open Data Directive (ODD) | Directive (EU) 2019/1024 that promotes the availability and reuse of public sector and publicly funded data across the EU. |
| Patients | Individuals who are the primary beneficiaries of health data sharing. They gain access to their own health records and may contribute to research through data altruism. |
| Policymakers | Authorities who design and implement regulations and policies. They use health data to inform evidence-based decisions and ensure ethical data governance. |
| Primary use of data | The use of health data for the original purpose for which it was collected, such as direct patient care. |
| Reusers | Researchers, academics and innovators who access anonymised or pseudonymised health data for secondary purposes such as scientific research, public health and innovation. |
| Secondary use of data | The reuse of health data for purposes other than direct care, such as research, innovation, policymaking and public health planning. |
| Use case | A scenario that illustrates how a specific user group interacts with the EHDS or open data, highlighting benefits, challenges and potential solutions. |
-([1]) Regulation (EU) 2025/327 of the European Parliament and of the Council of 11 February 2025 on the European Health Data Space and amending Directive 2011/24/EU and Regulation (EU) 2024/2847, OJ L, 2025/327, 5.3.2025, ELI: https://eur-lex.europa.eu/eli/reg/2025/327/oj/eng.
([2]) Directive (EU) 2019/1024 of the European Parliament and of the Council of 20 June 2019 on open data and the re-use of public sector information (recast), OJ L 172, 26.6.2019, p. 56, ELI: https://eur-lex.europa.eu/eli/dir/2019/1024/oj/eng.
([3]) European Commission, ‘A European strategy for data’, European Commission website, 9 April 2025, https://digital-strategy.ec.europa.eu/en/policies/strategy-data.
([4]) Publications Office of the European Union, ‘Pioneering the EU’s sector-specific data spaces: The European Health Data Space’, data.europa.eu website, 19 September 2024, https://data.europa.eu/en/publications/datastories/pioneering-eus-sector-specific-data-spaces-european-health-data-space.
([5]) European Commission, ‘Digital health and care’, European Commission website, https://health.ec.europa.eu/ehealth-digital-health-and-care/digital-health-and-care_en.
([6]) Commission Implementing Regulation (EU) 2023/138 of 21 December 2022 laying down a list of specific high-value datasets and the arrangements for their publication and re-use, OJ L 19, 20.1.2023, p. 43, ELI: http://data.europa.eu/eli/reg_impl/2023/138/oj.
([7]) European Commission: Directorate-General for Communications Networks, Content and Technology and PwC, Identification of data themes for the extensions of public sector high-value datasets – Final study, Publications Office of the European Union, Luxembourg, 2023, https://data.europa.eu/doi/10.2759/739414.
([8]) Commission Implementing Regulation (EU) 2023/138 of 21 December 2022 laying down a list of specific high-value datasets and the arrangements for their publication and re-use, OJ L 19, 20.1.2023, p. 43, ELI: http://data.europa.eu/eli/reg_impl/2023/138/oj.
([9]) Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions – A European strategy for data, COM(2020) 66 final of 19 February 2020, https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52020DC0066.
([10]) European Commission, ‘A European strategy for data’, European Commission website, 9 April 2025, https://digital-strategy.ec.europa.eu/en/policies/strategy-data.
([11]) European Commission, ‘Common European data spaces’, European Commission website, 9 July 2025, https://digital-strategy.ec.europa.eu/en/policies/data-spaces.
([12]) Regulation (EU) 2022/868 of the European Parliament and of the Council of 30 May 2022 on European data governance and amending Regulation (EU) 2018/1724 (Data Governance Act) OJ L 152, 3.6.2022, p. 1, ELI: http://data.europa.eu/eli/reg/2022/868/oj.
([13]) Regulation (EU) 2023/2854 of the European Parliament and of the Council of 13 December 2023 on harmonised rules on fair access to and use of data and amending Regulation (EU) 2017/2394 and Directive (EU) 2020/1828 (Data Act), OJ L, 2023/2854, 22.12.2023, ELI: https://eur-lex.europa.eu/eli/reg/2023/2854/oj/eng.
([14]) Regulation (EU) 2025/327 of the European Parliament and of the Council of 11 February 2025 on the European Health Data Space and amending Directive 2011/24/EU and Regulation (EU) 2024/2847, OJ L, 2025/327, 5.3.2025, ELI: http://data.europa.eu/eli/reg/2025/327/oj.
([15]) European Commission, ‘European Health Data Space Regulation (EHDS)’, European Commission website, https://health.ec.europa.eu/ehealth-digital-health-and-care/european-health-data-space-regulation-ehds_en.
([16]) Article 1 of Regulation (EU) 2025/327 of the European Parliament and of the Council of 11 February 2025 on the European Health Data Space and amending Directive 2011/24/EU and Regulation (EU) 2024/2847, OJ L, 2025/327, 5.3.2025, ELI: https://eur-lex.europa.eu/eli/reg/2025/327/oj/eng.
([17]) European Commission, ‘Electronic cross-border health services’, European Commission website, https://health.ec.europa.eu/ehealth-digital-health-and-care/digital-health-and-care/electronic-cross-border-health-services_en.
([18]) European Commission, ‘EU4Health programme 2021–2027 – a vision for a healthier European Union’, European Commission website, https://health.ec.europa.eu/funding/eu4health-programme-2021-2027-vision-healthier-european-union_en.
([19]) European Commission, ‘Horizon Europe’, European Commission website, https://commission.europa.eu/funding-tenders/find-funding/eu-funding-programmes/horizon-europe_en.
([20]) Finnish Innovation Fund Sitra, ‘Second Joint Action Towards the European Health Data Space – TEHDAS2’, tehdas.eu website, https://tehdas.eu/.
([21]) European Commission, ‘Health Data Access Bodies – Community of Practice’, European Commission website, https://health.ec.europa.eu/ehealth-digital-health-and-care/ehds-action/projects-supporting-ehds/health-data-access-bodies-community-practice_en.
([22]) Directive (EU) 2019/1024 of the European Parliament and of the Council of 20 June 2019 on open data and the re-use of public sector information (recast), OJ L 172, 26.6.2019, p. 56, ELI: http://data.europa.eu/eli/dir/2019/1024/oj.
([23]) European Commission, ‘INSPIRE Knowledge Base: Policy context’, INSPIRE Knowledge Base website, https://knowledge-base.inspire.ec.europa.eu/policy-context_en.
([24]) Publications Office of the European Union, ‘Datasets by theme: Statistics’, data.europa.eu website, 5 January 2025, https://data.europa.eu/catalogue-statistics/currentState/category?locale=en.
([25]) Publications Office of the European Union, ‘The value of health data and its role in Europe’, data.europa.eu website, 22 September 2022, https://data.europa.eu/en/publications/datastories/value-health-data-and-its-role-europe.
([26]) Ritoré, Á., Jiménez, C. M., González, J. L., Rejón-Parrilla, J. C., Hervás P. et al., ‘The role of Open Access Data in democratizing healthcare AI: A pathway to research enhancement, patient well-being and treatment equity in Andalusia, Spain’, PLOS Digital Health, e0000599, 2024, https://journals.plos.org/digitalhealth/article?id=10.1371/journal.pdig.0000599.
([27]) Commission staff working document – Impact assessment report – Accompanying the document ‘Proposal for a regulation of the European Parliament and of the Council on the European health data space’, SWD(2022) 131 final of 3 May 2022, https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52022SC0131&qid=1753366934773.
([28]) Puussaar, A., Johnson, I. G., Montague, K., James P. and Wright P., ‘Making Open Data Work for Civic Advocacy’, in: Karahalios, K., Monroy-Hernández, A., Lampinen, A. and Fitzpatrick G. (eds), Proceedings of the ACM on Human-Computer Interaction, Association for Computing Machinery, New York, November 2018, pp. 1–20, https://doi.org/10.1145/3274412.
([29]) PatientsLikeMe, PatientsLikeMe website, https://www.patientslikeme.com/.
([30]) EIT Health, ‘Implementing the European Health Data Space Across Europe’, April 2024, https://eithealth.eu/wp-content/uploads/2024/04/EIT_Health_ThinkTank_Implementing_the_EHDS_across_Europe_23.04.24.pdf.
([31]) Derycke, P., ‘HealthDCAT-AP’, 22 December 2023, https://healthdcat-ap.github.io/.
([32]) See for example the Explanatory Memorandum of the Proposal for a regulation of the European Parliament and of the Council on the European health data space COM(2022) 197 final of 3 May 2022, https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52022PC0197.
([33]) Per Member State, specific HDABs are being developed that manage data requests or give out data access applications.
([34]) Ministry of Ecological Transition and Territorial Cohesion of France and the Ministry of Energy Transition of France, ‘Green Data for Health’, Green Data for Health website, https://gd4h.ecologie.gouv.fr/https://gd4h.ecologie.gouv.fr/en.
([35]) Ministry of Ecological Transition and Territorial Cohesion of France and the Ministry of Energy Transition of France, ‘Call for Projects from Green Data for Health and the Health Data Hub’, Green Data for Health website, https://gd4h.ecologie.gouv.fr/https://gd4h.ecologie.gouv.fr/enhttps://gd4h.ecologie.gouv.fr/appel-a-projets.
([36]) See for example open data from the Italian Bureau of Statistics (https://avvisi.istat.it/IdotStat/). It provide datasets on topics such as healthcare expenditure and status of healthcare facilities. For an example of a dataset, see the following on public and private hospitals and their activities: https://esploradati.istat.it/databrowser/#/en/dw/categories/IT1,Z0810HEA,1.0/HEA_SERVICES/DCIS_OSPED/IT1,43_967_DF_DCIS_OSPED_1,1.0. For Austria, the following dataset could be an example: https://www.statistik.at/en/statistics/population-and-society/health/healthcare-and-expenditure/inpatient-healthcare-hospital-discharges.
([37]) Ministry of Ecological Transition and Territorial Cohesion of France and the Ministry of Energy Transition of France, ‘Green Data for Health’, Green Data for Health website, https://gd4h.ecologie.gouv.fr/.
([38]) As the EHDS legislation came into force in March 2025, in March 2027 the first obligations should be in practice.
([39]) Finnish Innovation Fund Sitra, ‘Second Joint Action Towards the European Health Data Space – TEHDAS2’, tehdas.eu website, https://tehdas.eu/.
